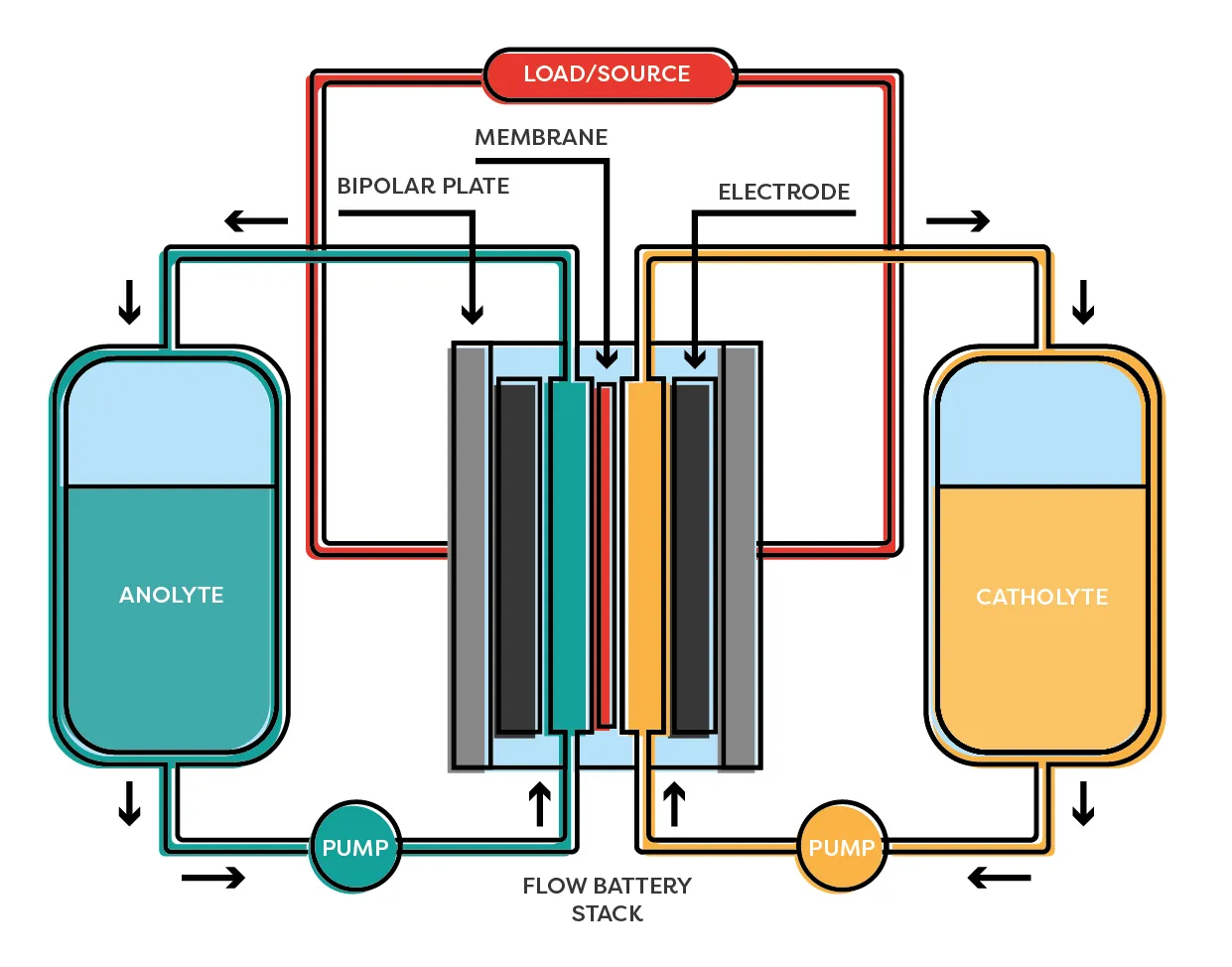
Redox-flow Battery
A redox-flow battery is an electrochemical system that stores energy and is rechargeable. It consists of two electrolytes – positive (catalytic) and negative (anolytic) – which are stored in two separate containers. In order to convert energy, the electrolytes pass through an electrochemical cell consisting of two semiconductor cells separated by a membrane. Each half cell contains a graphite felt electrode, on which a partial redox reaction takes place.
A redox-flow battery technology is similar to both a fuel cell and a battery where liquid energy sources are used to generate electricity and can be charged in the same system. Flow batteries are ideal for applications such as the integration of renewable energy sources, as industrial back-up power or temporary storage to stabilize the grid. One of the biggest advantages of the floating batteries is that they can be recharged almost immediately by changing the electrolyte fluid and at the same time recovering the used material for recharging.
We offer graphite felt electrodes for redox-flow batteries as we can supply specialized graphite products that meet the highest standards of efficiency and quality. Our graphite materials are distinguished by their electrical conductivity, purity and corrosion resistance – leading to a significant increase in performance as well as reliability in energy storage.
Thanks to our knowledge, many years of experience and close cooperation with our customers, we are able to constantly improve our products, providing our customers with the highest quality products.
Contact us for more information.